
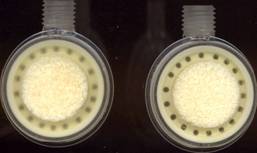
The First Resterilizable And Re-usable Agar Dish In The World
A product innovation with cost saving and safety benefits inside microbiological laboratory practice. With the help of an intelligent re-use conception thousands tons of waste and precious valuable plastic material could be avoided or saved.
After the laboratory use the Repetri dishes will be arranged in a special magazin and during the sterilization procession the contaminated agar will leave the Repetri dish and will be sterilized within the same cycle.
With the use of the Repetri dish you are avoiding any contaminated waste, which has to be elimated with the use of the normal Petri dishes in a cost intensive way (transport and destruction of contaminated extra waste under controlled conditions).
The Repetrri dishes are fit for the future, they could be resterilized in temperature ranges from about 180°C, where you can destroy high resistent microbiological structures in a safe and effectiv way.
The First Resterilizable Re-usable Dialyzer Of The World
The principle of Re-use from dialyzers in the nephrological fielding is a common practise especially in the USA.
One of the biggest disadvantages is the requirement of a validated disinfection cycle after the use, which is not 100 % under control.
One of the key goals for this very special development was the construction of a new dialyzer which could be resterilized (up to 50 cycles at 134° in a filled condition) and equipped with a special elctronic chip for a clear and safe documentation of all re-use procedures.
Instead of using 150 Dialyzers/patient/year you have only to introduce 3 Dialyzers /patient /year. These is a fundamental base for an enormous cost and waste reduction potential inside the dialysis treatment procedures.
Drying Procession For Synthetic Hollow Fiber Membranes With The Support Of Microwave Energy
General aspects
During the procession of synthetic hollow fiber membranes for medical filters (e.g. dialyzer) the leak test is one of the most important steps. The physical characteristic of the membranes (with the well-known air permeablity under dry conditions) makes it necessary to wet the membrane and to dry it back after passing the physical leak test.
If we take a more intensive look on the back drying of a membrane inside a dialyzer, you have to install first a pre-drying period, to remove bigger amounts of residuel fluids from the both dialyzer compartments, before using hot air or microwave energy
Drying procedure
Substances with a specific dielectric coefficient could react during microwave exposure. The molecules will be affected, they are vibrating and together with the internal friction a frictional heat will be built, which could warm up rigid materials or fluids (e.g. water).
One or more dialyzers which has passed a pre-drying period (with a specific constant residuel fluid level) will be adapted into a microwave equipment. The blood and dialysate compartments will be connected manually or automaticly. In combination with the microwave equipment you need a specific, constant and controlled amount of air stream running through every dialyzer.
The equipment must be special adapted for the installation in a dialyzer production. The most important parameter is the homogeneous perfomance distribution of the microwave energy, which could guarantee a concurrency and constant drying result inside several dialyzers. With the controlling of the energy stream inside the drying room you can reach a gentle cycle which can keep the perfomance level of the membrane.
The humidity leaving the dialyzer will be caught by a condensate trap and seperated from the exit air.
Technical datas
Drying time (depending on type of dialyzer) 4 ... 8 min
Dialyzer/cycle 1 ... 5 pcs.
Weight tolerance in several dialyzers/cycle < 2 g
Remark:
The drying time is one of the most important parameters for the final weight of the dialyzer. In dependency of the sterilization method, you can look for optimized performance conditions in adapting the drying time to the suitable humidity values inside the membrane. A very important influence in the final humidity result and their constant values is given by the constance of the entrance weight values (after humidification and predrying) of every dialyzer !
Laser Sealing Of Bundle Edges From Synthetic Hollow Fiber Membranes Used For Medical Filters
General Aspects
Dialyzers are used in the meidcal fielding to remove water and other substances from the body when the kidney shows malfunctions. The base of a successful treatment is a high perfomance dialyzer which will provide the cleaning of the blood with simultaneous retention of blood cells and proteins, through physical applications like ultrafiltration, diffusion or osmose.
Cellulosic or synthetic hollow fiber membranes will be actually used. You have a blood flow inside the hollow fiber membranes and a streaming around the fibers with a special fluid. Inside the dialyzer you have two seperated "rooms" which are only seperated with a less nm thick membrane. For the production of such dialyzers you need hollow fiber bundles built from 5.000 to 15.000 individuel fibers, which has to be glued exactly in a specific housing.
Potting of synthetic hollow fiber membranes
Synthetic hollow fiber membranes are generally spoken in the most of the cases air-permeable in a dry condition, the cellulosic membranes not.
The production of a dialyzer is a very difficult multi step process. The ends of the fibers and the space between them has to be potted with a medical approved resin (mainly used Polyurethan - PUR). The inner rooms of the hollow fibers must be complete free of PUR, in order to provide an easy blood flow. The goal can be achieved by centrifuging the PUR and by a final cutting of the fiber ends afterwards. One step in in this manufacturing procedure is imortant: the sealing of the hollow fiber ends, to prohibit the entering of PUR into the lumen of the fibers. For the solution of the problem you can use a two step potting with special viscosity PUR materials, or you can use a old fashioned hot wire technique or a new invented laser procession.
Principle technology
The goal for the innovated development step, was to look for a contactless laser treatment of the hollow fibers. We have to bring up a specific melting temperature concentrated on the ends of the hollow fibers within a very short time. We have also to guarantee that the fibers will not be bonded directly to each other; because the potting glue have to get the possibility to move around all fibers and to fix a constant distance to each other.
Technical Datas
Performance about 250 W
Processing time 4 ... 6 s
Material loss in the bundle edges 3 ... 4 mm
Advantages of the procedure
The new procedure can easily fulfill the very strong regulations for medical production steps (validation, calibration, process stability a.s.o.m.) and is a very safe and cost optimized production step.
After passing the laser sealing, you could use parallelly the production equipment for cellulosic membranes, you normally don't need a parameter change between cellulosic and synthetic membranes.
After the cutting you could not see any contraction cavities inside the PUR cutting area, and the laser use will help you in minimizing the fiberloss during the heat treatment in comparison to the hot-wire tecnique.

Development Of New Filtration Generations For Different Filtration Procedures
We have developped new housing conceptions for a more efficient performance improvement inside different medical filters.
Rectangular constructions, multi chamber systems, arched housing variations are only a small range of different housing types, which we have manufactured for different project decisions within the last months and years.
The filter housings, prepared with different geometric construction modifications in a steam autoclavable plastic material, were tested for different applications in blood separation and purification, concentration cells (gas + fluids) and biological cell marking in different laboratories. The results were used as fundamental decision base for different national and international investment groups.

Newest Generation Of Plastic Sterile Containers
The newest achievement of the development team
... an intelligent thermal opening and closing unit. The system is reacting during the steam sterilization procedure by opening after the start of the prevacuum steps and by closing after the drying processing.
It's a brillant solution for the use in resterilizable plastic containers, you could eliminate the old fashioned filter exchange. We have also included a new closing mechanism and an innovative container gasket.
The system will be completed by a special sterilization programme which fits to the key autoclave types.
|